What we do
The INBRAIN Laboratory conducts basic and translational research to understand, measure, and improve the neurobiological processes that shape human performance, adaptation, and resilience in health and disease. Our work focuses on brain–body interactions in real-world contexts, examining how neural systems adapt to stress, training, and injury, with the goal of translating mechanistic insight into meaningful improvements in physical and cognitive function.
INBRAIN employs integrative, multimodal experimental approaches including non-invasive brain stimulation, neuroimaging, electrophysiology, wearable sensing, biomechanics, biochemistry, and psychometrics. These methods are paired with advanced analytical techniques such as graph theory and machine learning to characterize complex neurobehavioral systems across scales.
Our research is highly interdisciplinary and collaborative, spanning academia, medicine, and military organizations, and engaging experts in physiology, biomechanics, neuroscience, psychology, biochemistry, statistics, mathematics, psychiatry, nutrition, and surgical specialties.
Current and recent efforts include leveraging neuromodulation to optimize cognitive and physical performance, investigating use- and pathology-dependent neuroplasticity, and refining human neuroscience methodologies. INBRAIN research has been supported by the Department of Defense, National Institutes of Health, UK Ministry of Defence, National Aeronautics and Space Administration, and the National Strength and Conditioning Association.
Core focus areas
A central focus of INBRAIN is how structure and function adapt with use, disuse, and injury. We study plasticity across multiple scales to identify mechanisms underlying loss of function and recovery, with the goal of informing targeted interventions, diagnostic and prognostic indicators, and brain-based therapies.
Plasticity in health, injury, and recovery
Behavioral function and recovery are not purely musculoskeletal or peripheral processes—the brain plays a critical role. By identifying how neural systems adapt to experience, injury, and disease, INBRAIN aims to improve recovery trajectories and facilitate more effective therapies.
Why it matters
-
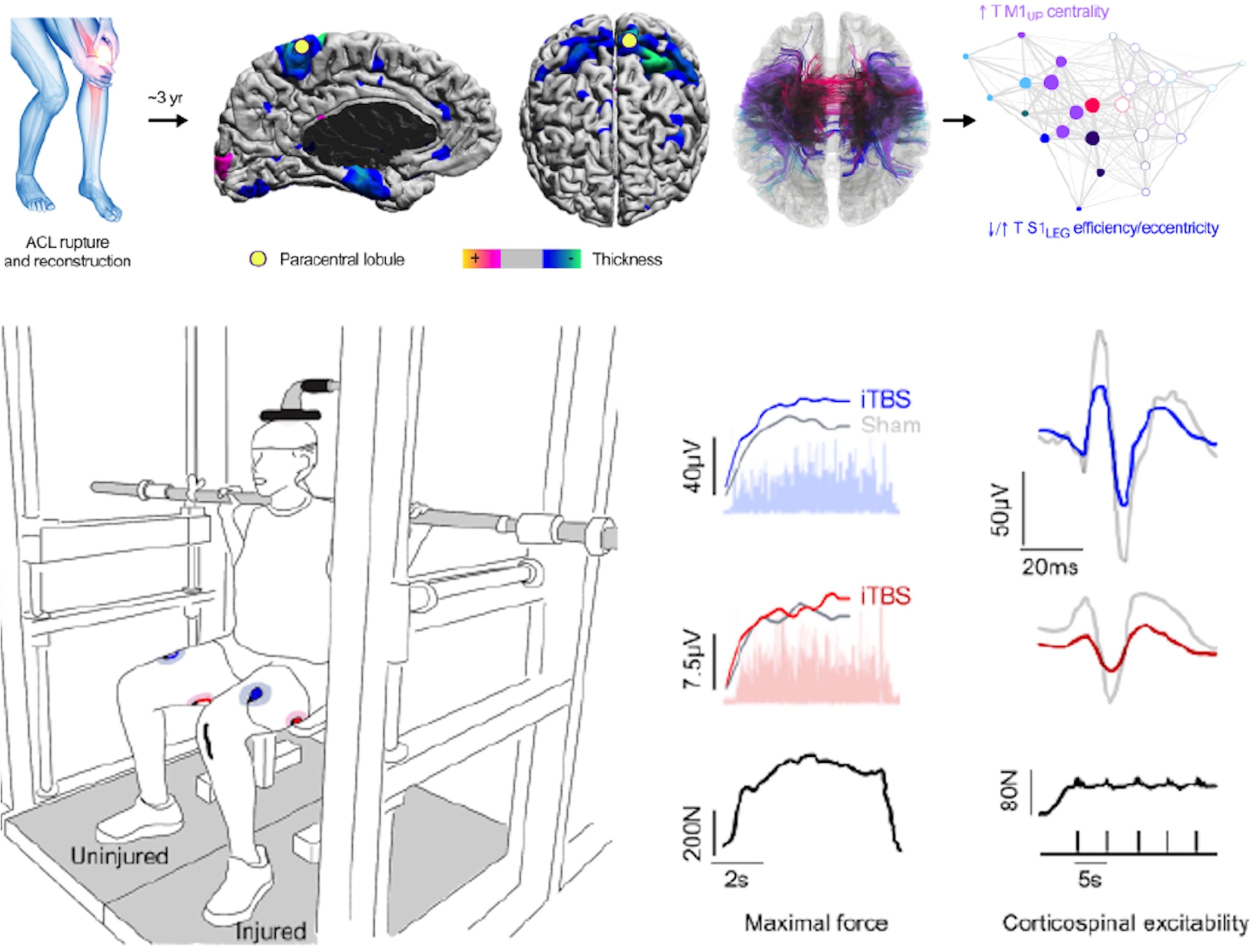
Combining multimodal neuroimaging with theta burst transcranial magnetic stimulation, we demonstrate that chronic musculoskeletal injury is associated with lasting, injury-specific alterations in corticomotor structure, excitability, and network organization. Importantly, individualized iTBS normalized both brain and muscle function years after injury, providing direct evidence that corticomotor plasticity contributes to persistent functional deficits and may represent a viable target for brain-based rehabilitation.
-
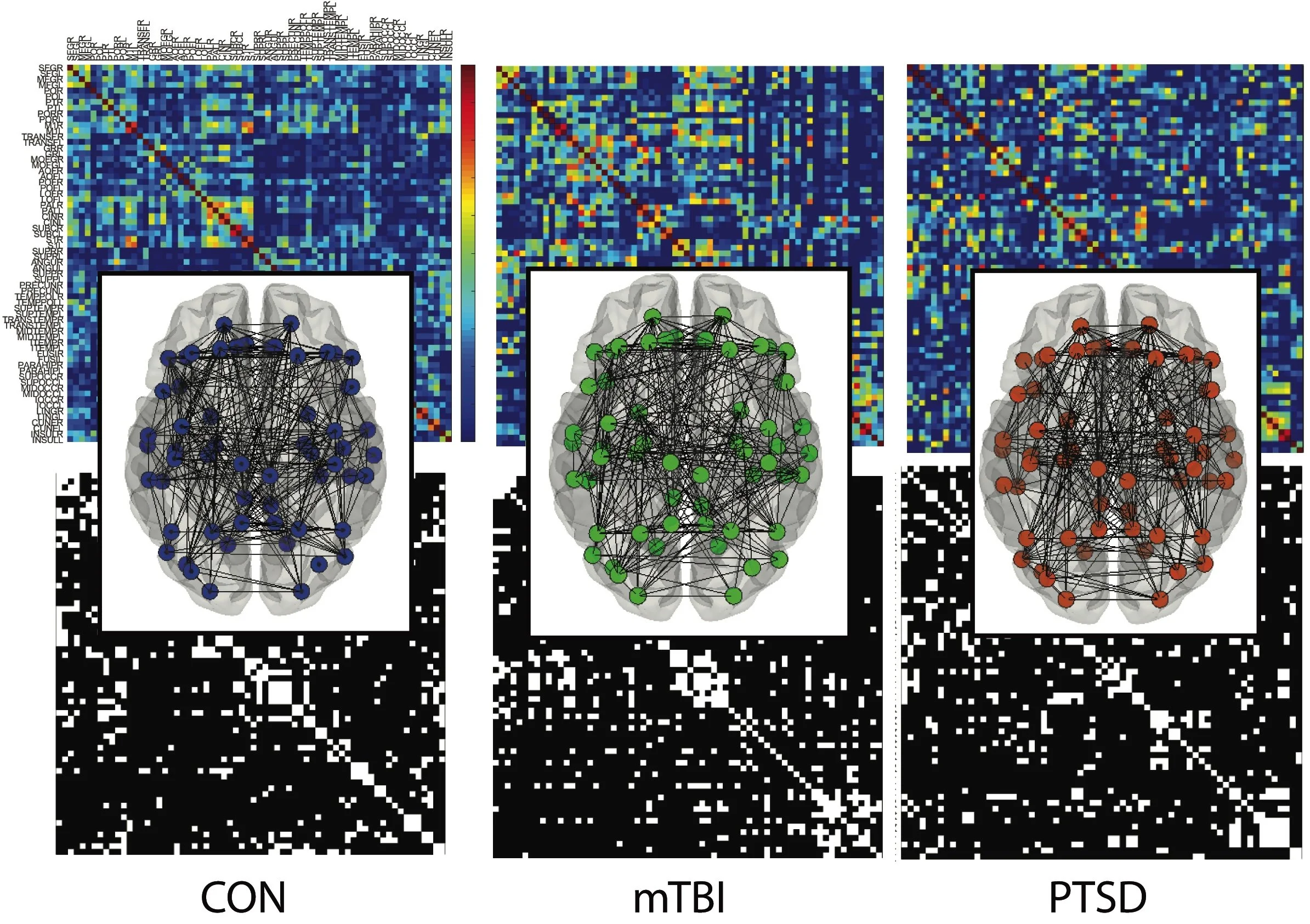
Using graph-theoretical analysis of cortical thickness–derived structural covariance networks, we identified distinct global and nodal brain network alterations in service members with mTBI, PTSD, and comorbid mTBI–PTSD. These findings reveal disorder-specific patterns of network segregation and fronto-limbic reorganization that may underlie shared and divergent emotional and cognitive symptoms in trauma-exposed populations.
-
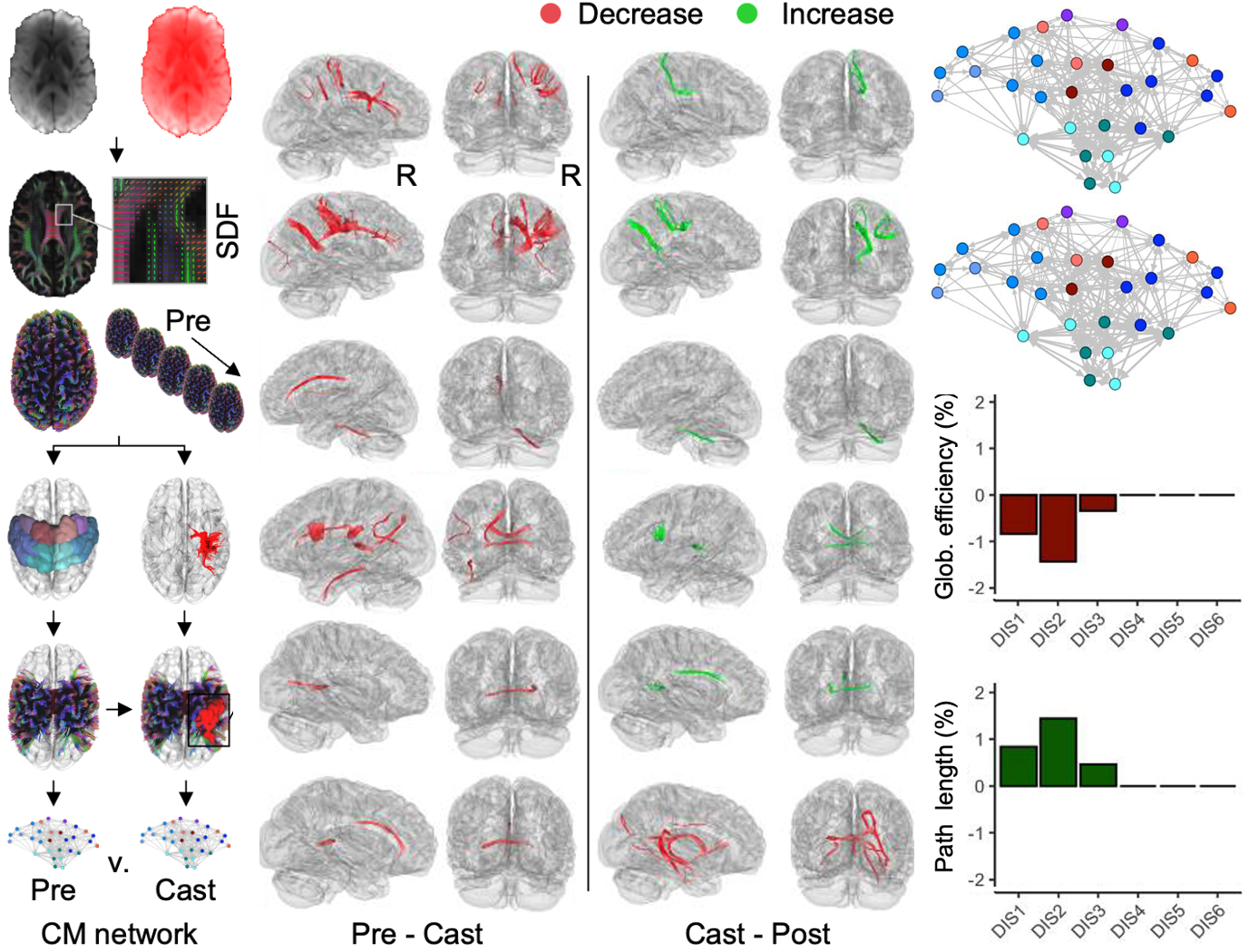
Using a dense, 21-day longitudinal design combining precision TMS and diffusion MRI, we show that short-term limb immobilization rapidly induces somatotopic, predominantly supraspinal reductions in corticospinal excitability and sensorimotor white-matter integrity that emerge within days and reverse quickly with remobilization. Motor imagery partially preserved strength and corticomotor signaling, highlighting the sensitivity—and modifiability—of early disuse-induced neuroplasticity
-
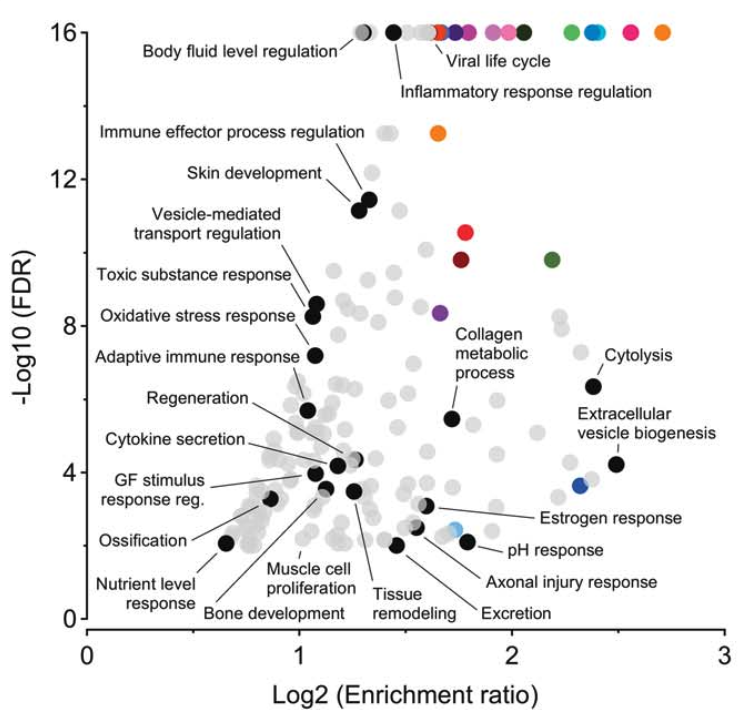
We optimized an advanced label-free quantitation mass spectrometry technique to identify urinary proteomic signatures that track adaptive changes in trabecular bone volume during U.S. Army Basic Combat Training. Our findings demonstrate the feasibility of noninvasive, scalable biomarkers of bone adaptation and resilience, with potential applications for early identification of individuals at risk for stress fractures and maladaptive skeletal responses.
Representative work
INBRAIN investigates how neural systems support physical and cognitive performance and adaptability. Our research focuses on emerging technological and behavioral interventions, realistic stressors, and body-body dynamics that govern performance, resilience, and recovery in healthy individuals and high-demand populations, including military personnel and athletes.
Neurobehavioral foundations of human performance and resilience
Understanding how the brain supports performance under pressure allows us to design better training strategies, identify markers of resilience, and develop interventions that help people perform safely and effectively in demanding environments.
Why it matters
-
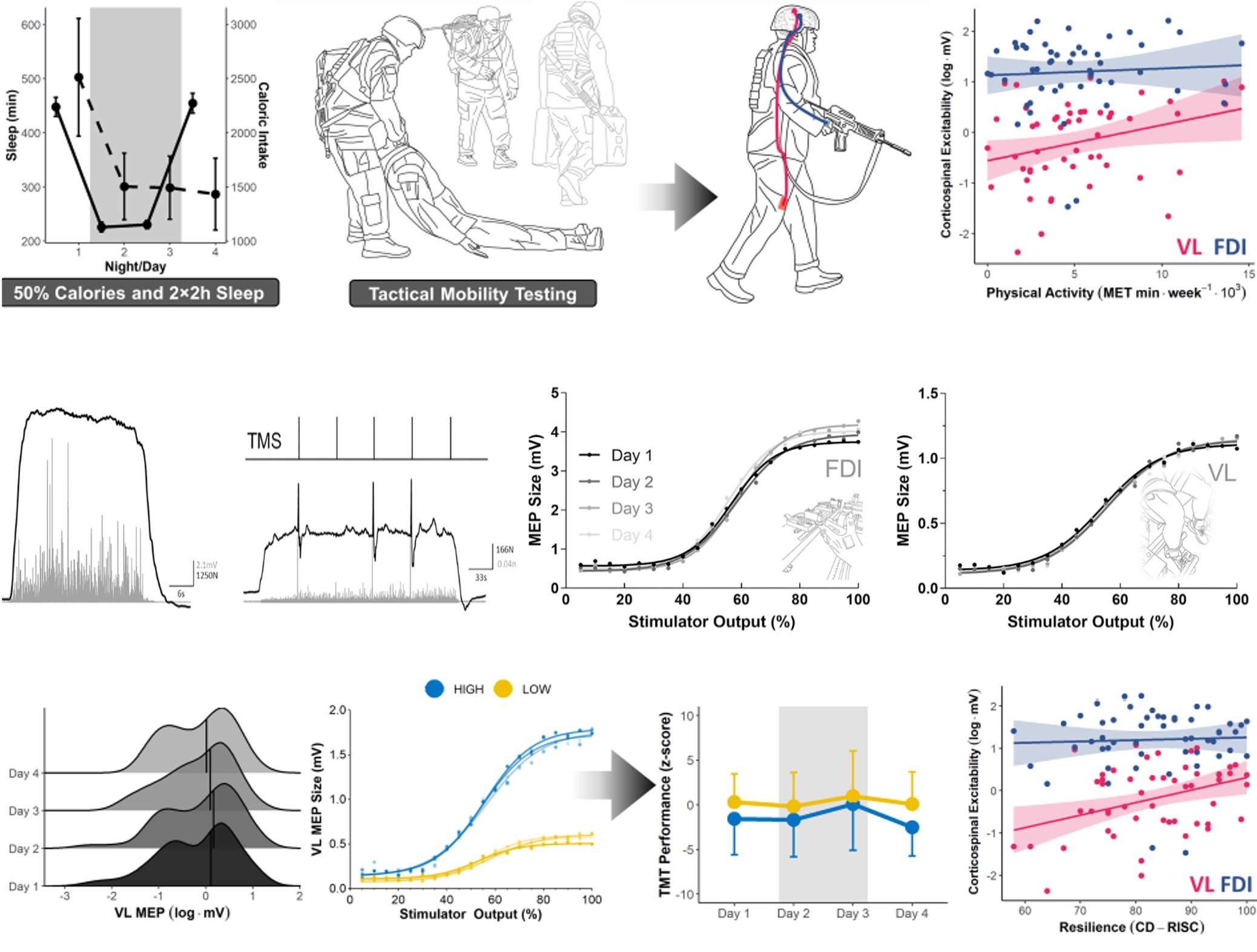
Using a realistic simulation of military operational stress, we show that individuals with higher lower-limb corticospinal excitability—reflecting greater habitual physical activity—exhibit superior physical performance and resilience despite severe sleep, caloric, and exertional stress. These findings link use-dependent neuroplasticity to real-world resilience and identify corticospinal excitability as a potential neural marker and intervention target for performance optimization under extreme conditions.
-
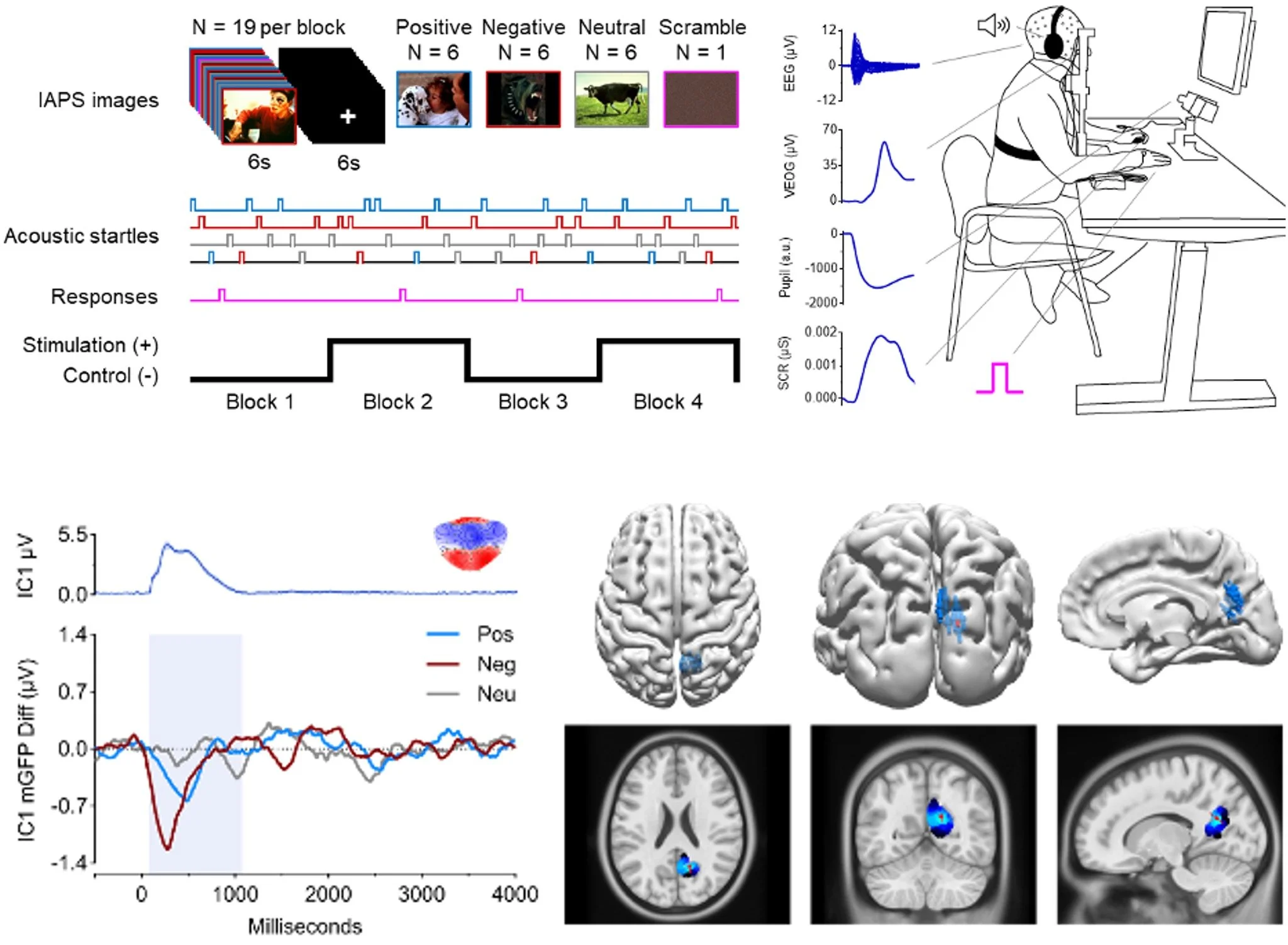
We demonstrate that daily transcutaneous auricular vagus nerve stimulation (taVNS) produces immediate and cumulative improvements in executive function—most evident during high-difficulty trials—alongside coordinated changes in autonomic arousal and flexibility. These effects were sex-dependent and persisted at rechallenge, informing dose, biomarker selection, and trial design while supporting taVNS as a safe, noninvasive approach for modulating cognition and autonomic function in healthy adults.
-
We examined how Compound K supplementation influences psychophysiological activity and performance before and after intense physical exercise stress. Compound K selectively modulated cortical activity linked to sustained attention and reduced stress-related sensory interference, suggesting a role in supporting neural efficiency during high-demand conditions.
-
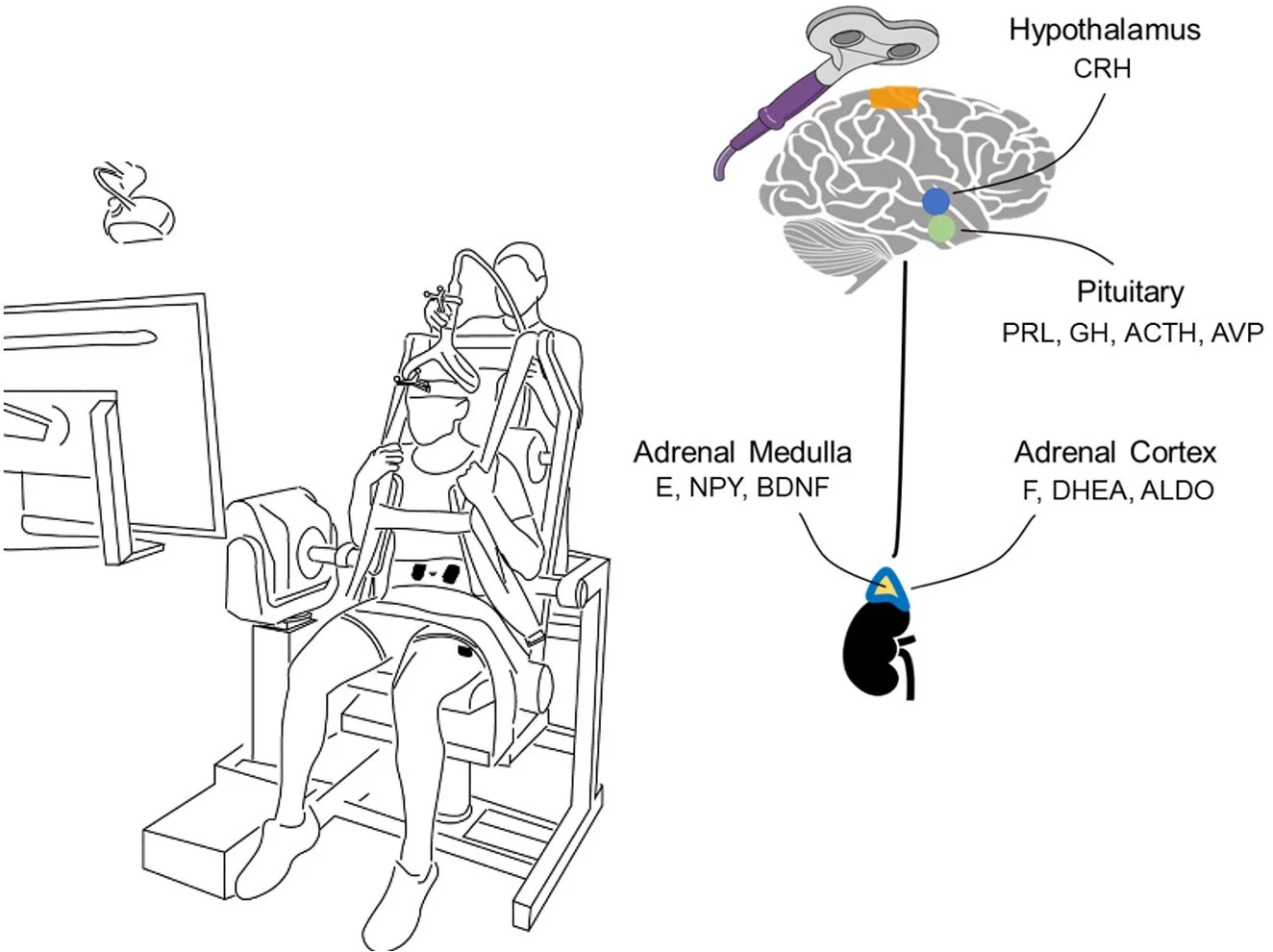
Building on the recent discovery of direct cortical projections to the adrenal medulla, this project tests whether precisely targeted non-invasive brain stimulation can modulate sympathoadrenomedullary activity. By combining individualized neuroimaging, optimized rTMS targeting of trunk motor and premotor regions, and multi-level physiological and behavioral outcomes, this work aims to establish a reproducible, mechanistically grounded approach for brain-based modulation of stress and performance systems in humans.
Representative work
INBRAIN develops, refines, and validates advanced tools for measuring and perturbing brain–body interactions in humans. Our work emphasizes rigor, reproducibility, and real-world applicability. We integrate causal and correlational approaches with modern analytical techniques to improve how human neuroscience is conducted in vivo.
Advancing human neuroscience techniques
Better tools lead to better science. By improving how we measure and stimulate human brain function, INBRAIN helps ensure that findings are reliable, interpretable, and ready to translate into clinical, performance, and operational settings.
Why it matters
-
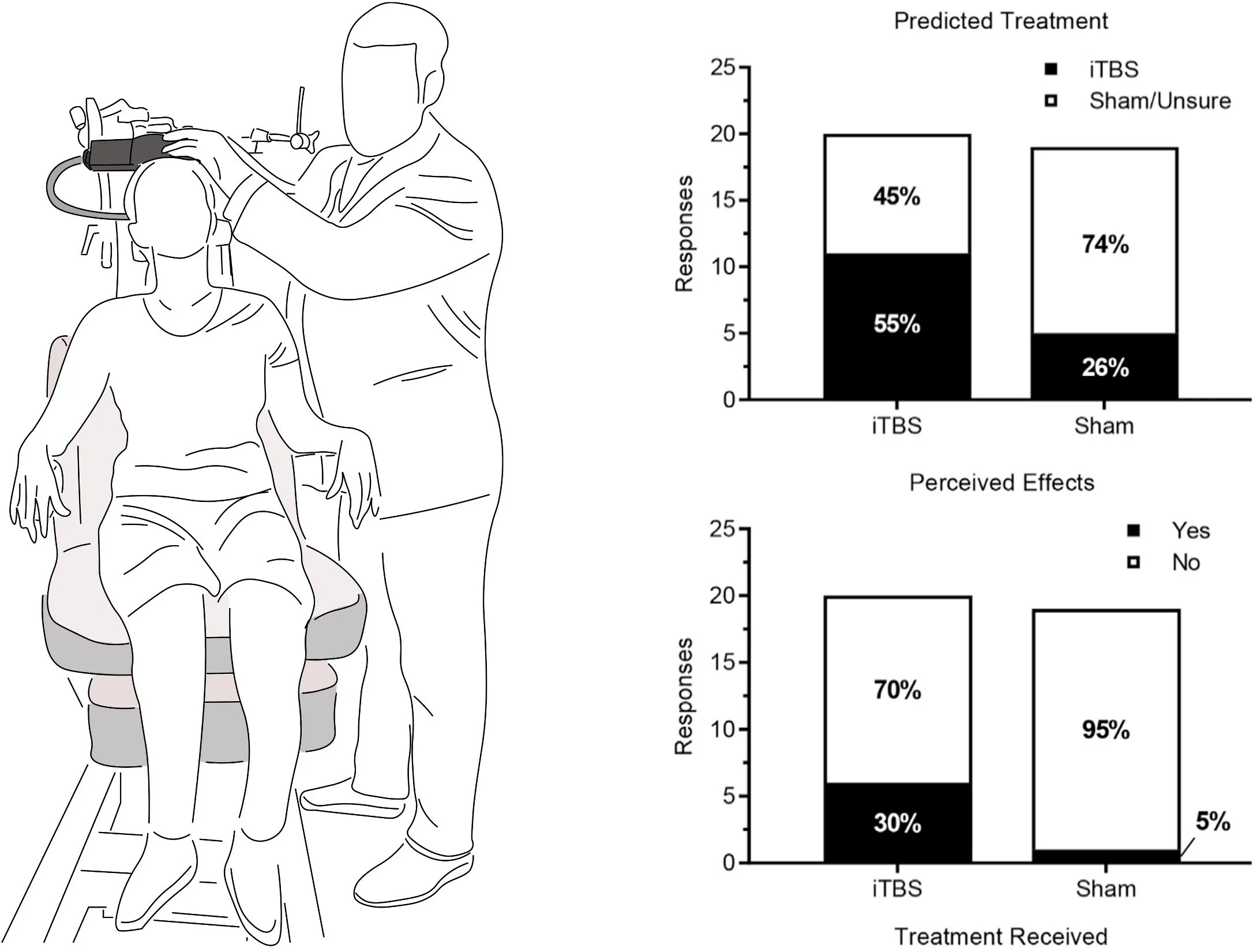
We examined whether participants could reliably distinguish active intermittent theta burst stimulation of motor cortex from a sham coil designed to mimic the sound, appearance, and scalp sensation of stimulation. This work directly tests a foundational assumption of neuromodulation trials and informs best practices for blinding, study design, and interpretation of behavioral and physiological outcomes.
-
We quantified the physiological extent of off-target corticospinal activation produced by double-cone TMS commonly used to stimulate lower-limb motor cortex, demonstrating substantial bilateral leg and unintended hand responses. These findings highlight the need to monitor and model off-target muscle activity to improve targeting accuracy, interpretation, and rigor in lower-limb TMS studies.
-
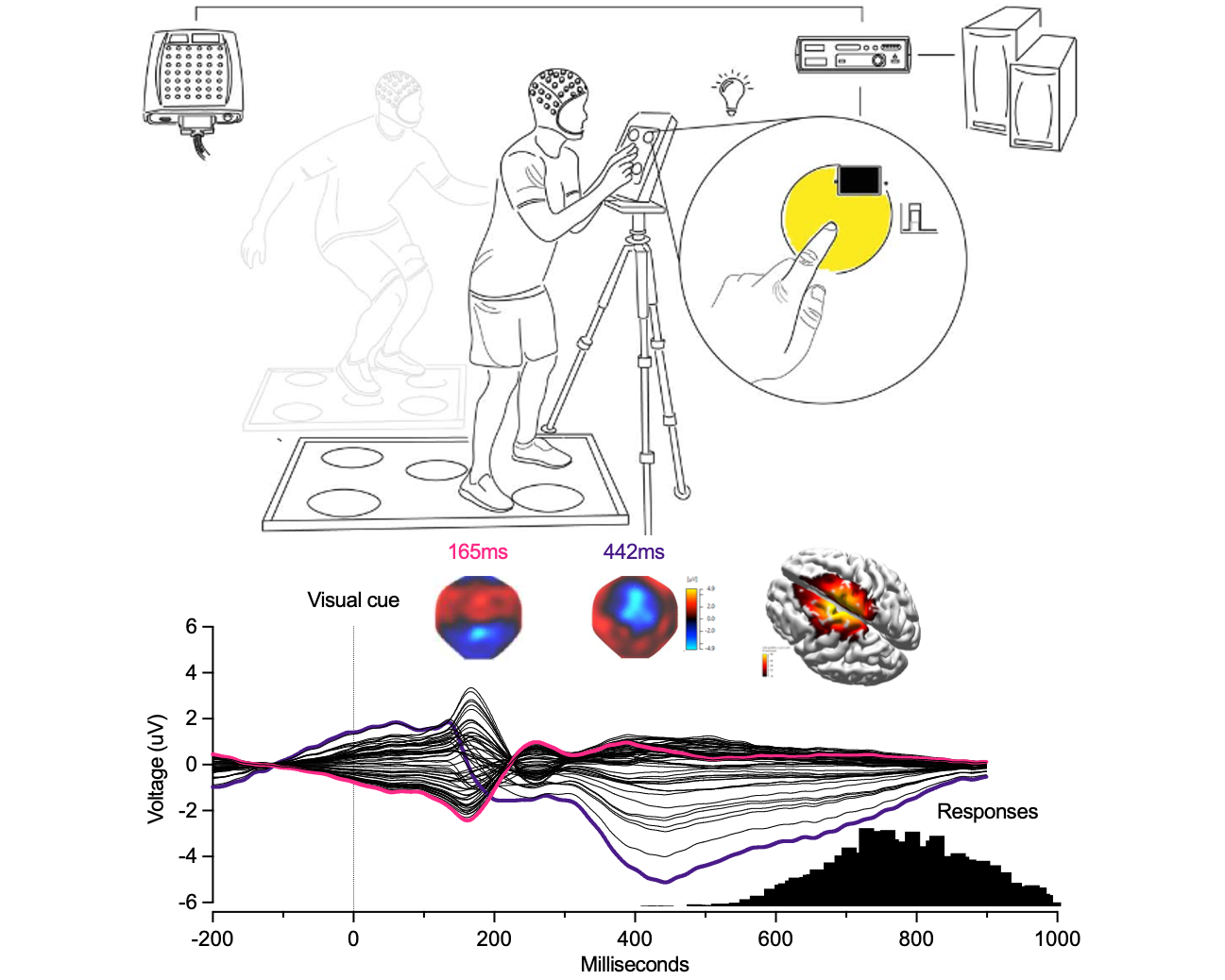
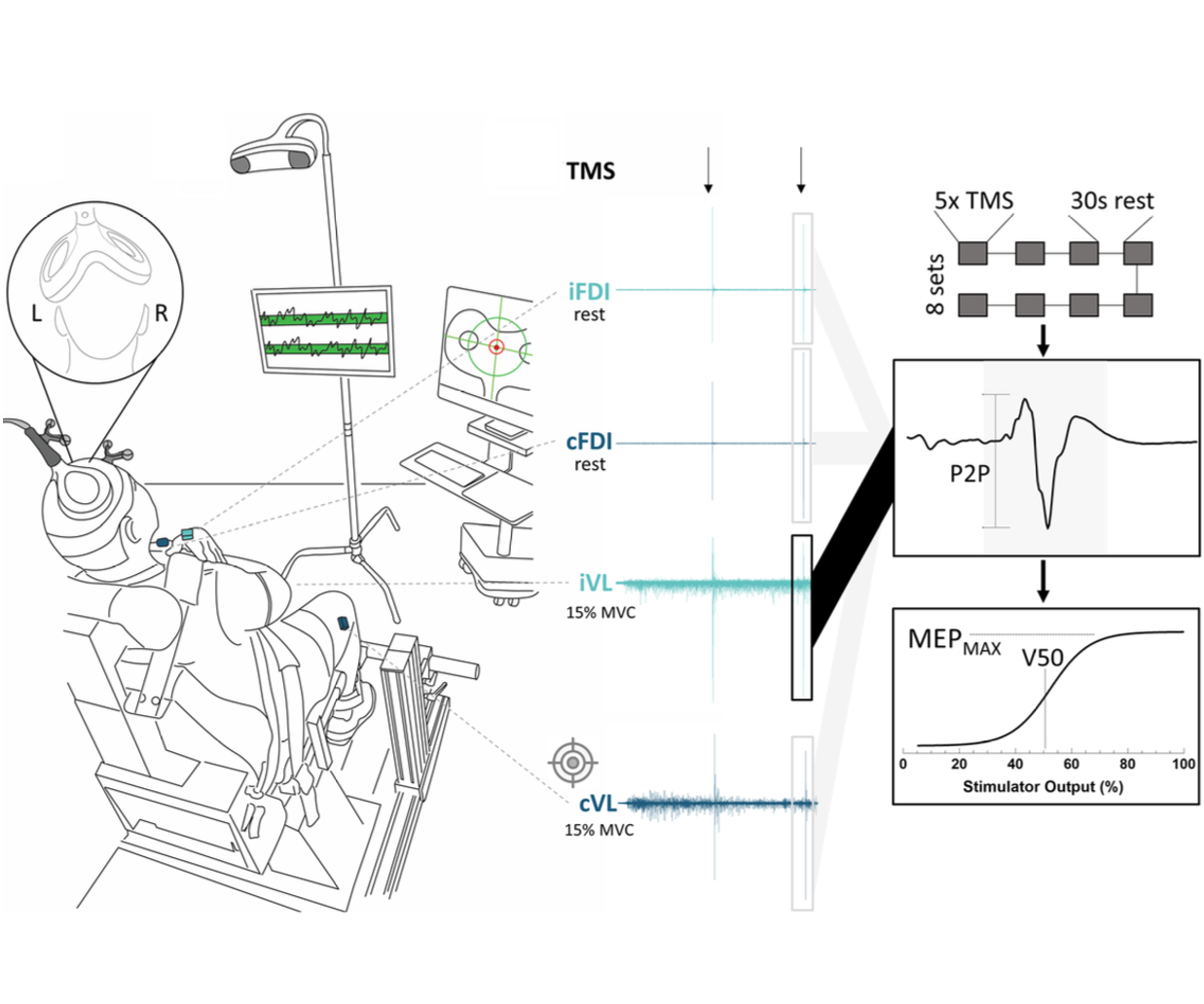
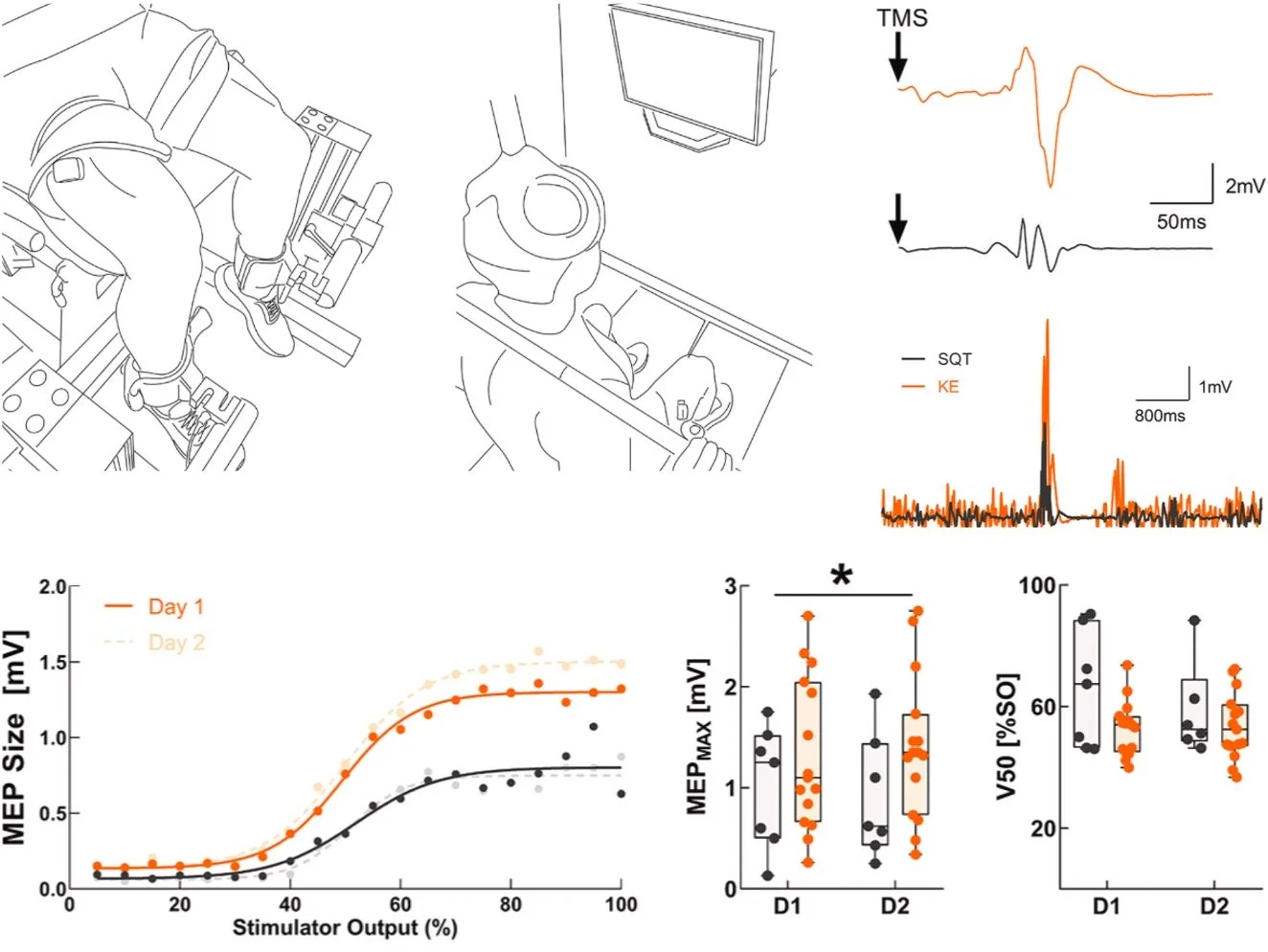
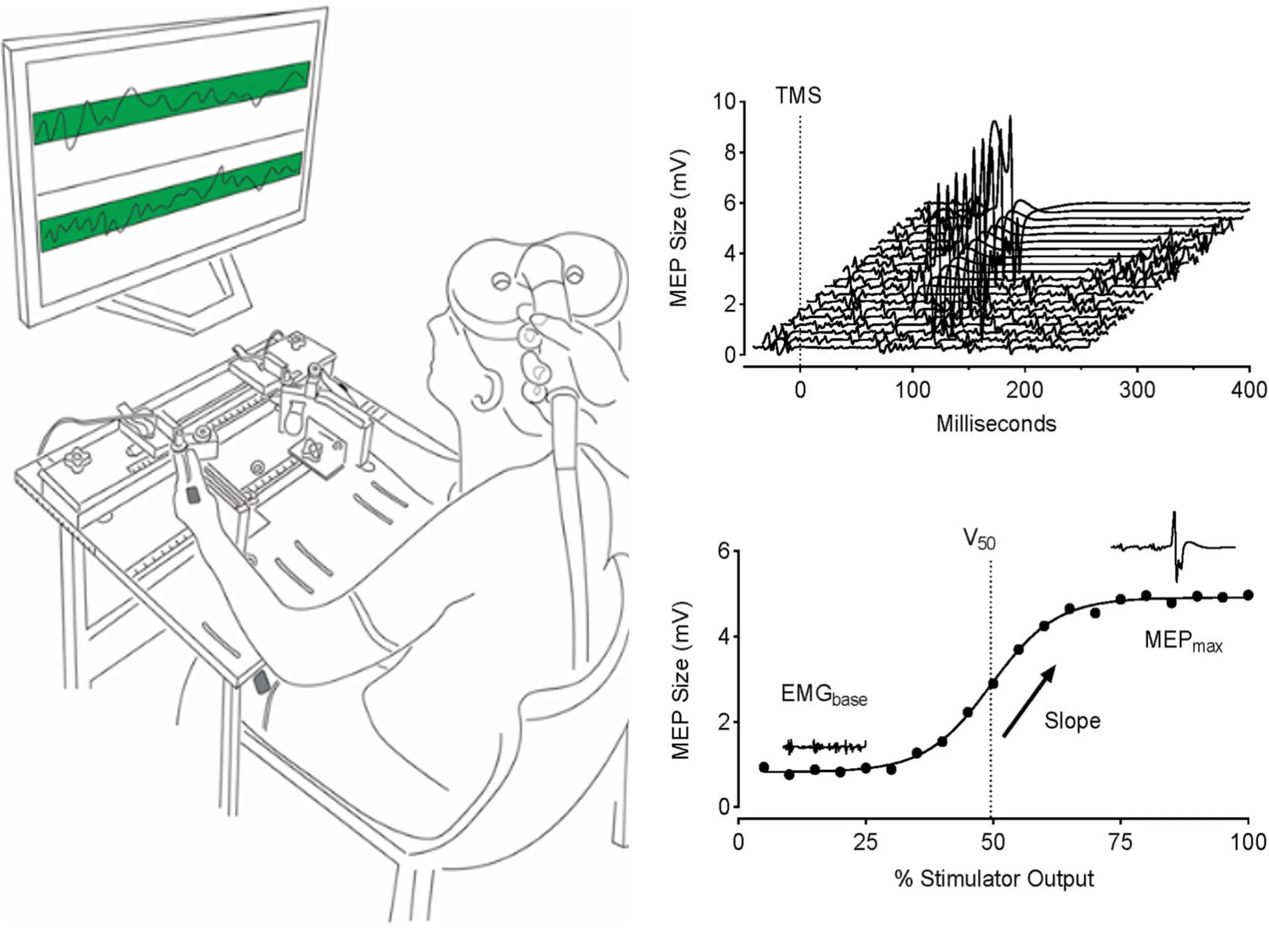
We compared the reliability of corticospinal excitability estimates for the vastus lateralis during traditional knee-extension tasks and more ecologically relevant squat-based tasks using stimulus–response curves. While squats better reflect real-world movement, knee extensions provided more reliable corticospinal measures, underscoring the need to balance ecological validity with measurement sensitivity when designing lower-limb TMS studies.
-
We evaluated the agreement and consistency of absolute- and relative-intensity TMS stimulus–response curves across upper-limb, lower-limb, and axial muscles. Our results show that both approaches yield comparable estimates of corticospinal excitability for key parameters, while highlighting muscle- and participant-specific factors that influence curve validity and should inform task and protocol selection in human TMS research.
-
We evaluated how ultra-short-term heart rate variability (HRV) metrics reflect autonomic regulation across rest, exercise, and recovery, and how measurement choices influence their physiological meaning. Our findings show that while many HRV measures are redundant, they capture state-specific autonomic information and are generally robust to preprocessing choices, supporting their cautious use as physiological biomarkers in human research.